The Hand Warmer Design Challenge. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch.

Ap Chemistry Files 4 Designing A Hand Warmer
Review the criteria for an ideal hand warmer from the Central Challenge.

. The Hand Warmer Design Challenge Investigation 12 CHALLENGE The ideal hand warmer increases in temperature by 20C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. The results provide a model for the guided-inquiry challenge which is to design an optimum hand warmer for consumer applications. DECEMBER 15 2010 Tis the season for cold fingers.
I put a paper clip on each fish and threw the whole mess into a fish tank. Working in groups each student group will be provided three different solids along with their costs. The Chemistry Of Hand Warmers.
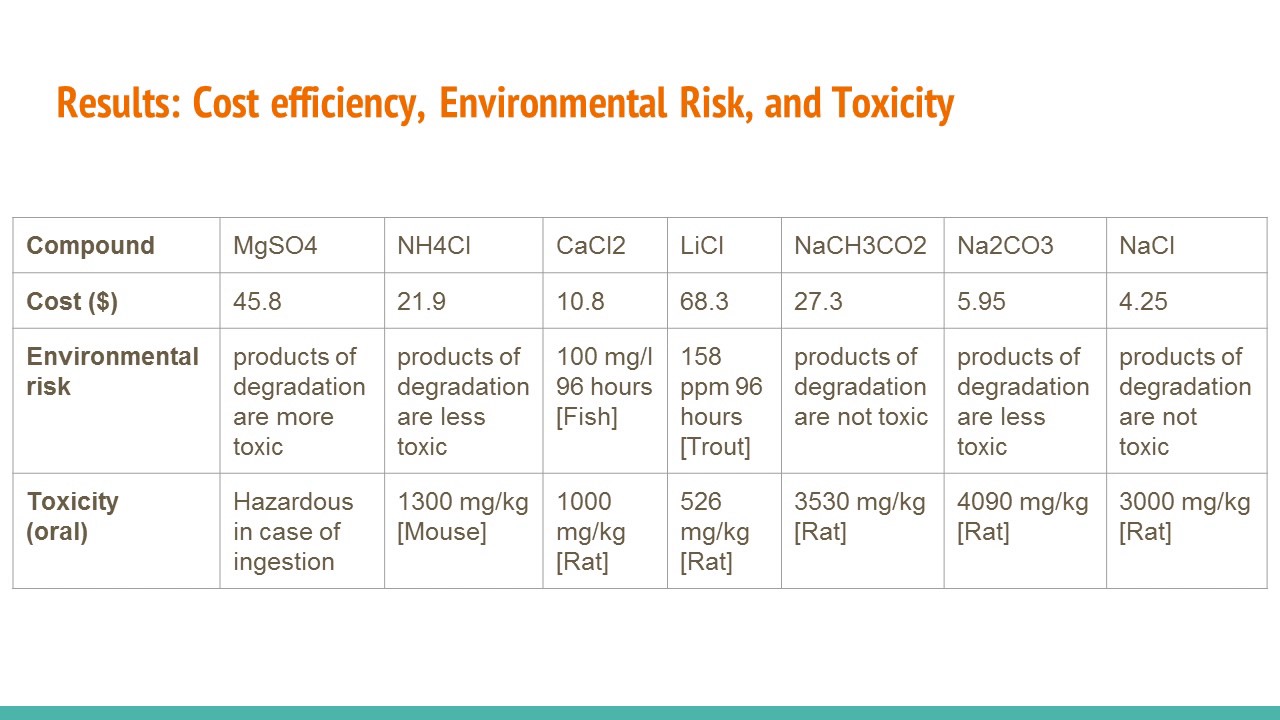
For each solid consider safety cost and environmental impact as well as the amount of heat released or absorbed. Learn Something New Every Day. Where Does the Heat Come From.
Set up investigation calculation data table as follows Useful tip. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds. Using Prezi Video for virtual sales presentations that convert.
The Hand Warmer Design Challenge. The Hand Warmer Design Challenge. The kids use the magnetic stir bar retriever I call the magic wand or fishing pole in this case to catch an acid fish.
Find the training resources you need for all your activities. The Challenge Design and build a safe effective economical and attractive hand warmer prototype to be used by school staff and students in the winter janitorial staff band sports teams security etc. Designing a Hand Warmer by Alexis Mabugat.
DAY 1 Part 2 only. The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a hot to a cold body Chemistry Designing A Hand Warmer Lab. Hand Warmer Challenge Lab Part B make the best judgement answer the Guided Inquiry and Design section in your spiral notebook.
For each solid consider safety cost and environmental impact as well as the amount of heat released or absorbed. This type of hand warmer tends to produce a more vigorous heat than the dry powder type of hand warmer but does not produce heat for quite as long. Design a procedure for the lab.
Lab hallsciencedsa weebly com designing a hand warmer pre lab answers tldr io the hand warmer design challenge prezi designing a hand warmer pre lab answers investigation 12 rancho hs enschool org designing a hand warmer lab chemistry pre lab 1 flashcards quizlet handwarmer design challenge lab helps 09 the hand warmer challenge ap. Explain it to your instructor before you proceed. The hand warmer you are designing needs to increase in temperature by 20 C.
Things to keep in. Q -786592 J Exothermic Reaction. Designing A Hand Warmer Ap Lab Answers.
Design and execute an experimental procedure to determine which of the ionic compounds is most suitable for use in a hand warmer. Design an optimum hand warmer for consumer applications. This needs to be included in your post lab.
Where Does the Heat Come From. To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer. The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a.
If youre stuck out in the cold for a few hours your mittens can only do so much. Chemistry Designing A Hand Warmer Lab Answers AP Chemistry Lab 10- Hand Warmer Design Challenge Big Idea 5 5A2. Be mindful of the safety procedures.
A type of reaction where energy is released in the form of light or heat. Answer question 1 for tomorrow. Programming brianThe Hand Warmer Design Challenge Prezi December 9th 2013 - The Hand Warmer Design Challenge without increasing too much as to burn a human hand will be the substance Pre Lab Questions Chemfax Labs Answers Hand Warmer bedale de May 26th 2018 - Read and Download Chemfax Labs Answers Hand Warmer Free Ebooks in PDF format.
Suitable for use in a hand warmer. Are the dissolving processes you carried out endothermic or exothermic or neither. Designing a Hand Warmer Lab Introduction.
Answer question 1 for tomorrow. Dissolve equal amount of each assigned solid in given amount of water with approximate mass of solution of 50g in each trial. How to get repeat customers.
233 would be required for a hand warmer that meets this requirement. Write down the procedure you will follow. Hand Warmer Challenge Post Lab.
Our hand warmer was an exothermic reaction since it released heat. Write a 200-400 word essay with all answers included. Safety Solids are eye and skin irritants.
Write down the procedure you will follow. AP MANUAL 2013 p. Be mindful of the safety procedures.
December 1st 2001 - ap chemistrythe hand warmer design challenge where does the heat come from lab 13 mattchemfax labs ANSWERS DESIGNING A HAND WARMER RUNNIN DE JUNE 18TH 2018 - READ AND DOWNLOAD CHEMFAX LABS ANSWERS DESIGNING A HAND WARMER FREE EBOOKS IN PDF FORMAT ANATOMY HISTOLOGY. Lab procedure must be approved by me before you continue. DAY 2 Lab Lab procedure must be approved by me before you continue.
Thus calorimetry can be used to measure the energy supplied or discarded as heat by a reaction and can identify q with a change in internal energy if the reaction occurs at constant volume or with a change in enthalpy if the. I cut out crude fish shapes from construction paper and taped the names and chemical formulas of the acids on them. Answer these in your lab notebook after the Purpose section 1 When chromium chloride CrCl 2.
Review the criteria for an ideal hand warmer from the Central Challenge. How to schedule fewer meetings and get more done. Determine the heat of solution for each solid and analyze the cost and safety information to propose a design for the best all-around hand warmer.
Science In Your Mittens. The goal of this lab is to design a safe effective environmentally benign and inexpensive hand warmer that will increase the temperature of water by 20 C but no more as quickly as possible with a volume of about 50 mL. Save it on a flash drive to use it tomorrow to upload to the Calibrated Peer Review Program.
48C - 245C 235C. The salt dissolves and the water warms. You will carry out an experiment to determine which substances in what.
The hand warmer lab is a lab from the new AP Chemistry Laboratory Notebook. Rank your solids from least to most expensive. 1 Measure out 2 separate samples of 1000 mL of distilled water.
Temperature Change T. Working in groups of four each student group will be. Where does the Heat come from.
Handwarmer Design Challenge Lab Helps April 7th 2019 - AP Chemistry Designing a Hand Warmer Lab Duration 10 49 Rebecca Poliner 1 219 views 10 49 8 Jobs Every Company will be Hiring for by 2020 Highest Paying jobs of future Designing.

Ap Inquiry 12 Hand Warmer Challenge

Ap Chemistry Hand Warmer Lab Youtube

Hand Warmer Design Challenge Bremen High School District 228

The Hand Warmer Design The Hand Warmer Design Challenge Where Does The Heat Come From Pre Lab Activity An Animation Showing The Dissolution Of An Course Hero
Designing A Hand Warmer Lab Youtube


0 comments
Post a Comment